GENOMICS BY VITROLIFE
Vitrolife now has exclusive distribution, development and commercialisation rights to Illumina’s preimplantation genetic testing business for IVF in EMEA and the Americas.
PGT-A
PGT-A (Preimplantation Genetic Testing for Aneuploidy), formerly referred to as PGS (Preimplantation Genetic Screening) determines the chromosomal status of an embryo by screening all 23 chromosome pairs prior to transfer in an IVF cycle.
Selecting the most viable embryo
Human cells typically have a total of 46 chromosomes – 23 from the mother (egg) and 23 from the father (sperm). When an embryo has an incorrect number of chromosomes, it is referred to as aneuploidy. Aneuploidy is one of the main causes of infertility and on average, approximately half of embryos in an IVF cycle are aneuploid, although this number may be higher in women of increased maternal age.1-3
Embryos with aneuploidy often fail to implant, and those that do implant often results in miscarriage.4,5 In rare cases a pregnancy with aneuploidy can sometimes leads to a live birth6, however in most cases these babies will have physical abnormalities and/or intellectual disabilities.

Preimplantation Genetic Testing for Aneuploidy (PGT-A)
During the IVF process, Preimplantation Genetic Testing for Aneuploidy (PGT-A) screens embryos to find those most likely to have the correct number of chromosomes. This may help to increase the chances of successful implantation and ongoing pregnancy while decreasing the chance of miscarriage.3,7-9
Meet the challenges with VeriSeq PGS Solution
PGT-A can be performed using next generation sequencing (NGS) using the fully-kitted VeriSeq PGS Solution on the Illumina MiSeq instrument. The VeriSeq PGS solution can screen all 24 chromosomes in as little as 12 hours for an accurate, efficient view of the number of chromosomes in an embryo.
Extensively validated
The VeriSeq PGS solution was extensively validated during in a large pre-clinical, multisite study. The product was validated for the detection of aneuploidy in preimplantation embryos, with both cell lines and embryo biopsy samples. The data set contained a good representation of chromosome losses and gains across all chromosomes which ensure a thorough evaluation of assay performance. VeriSeq PGS reliably detected chromosome aneuploidies in low DNA input samples across multiple sites, whether from biopsy of a single cell or a few cells.
Peer-Reviewed Publication
Fiorentino et al., (2014)10 performed a validation of the VeriSeq PGS Solution to investigate its applicability to PGT-A. Karyotypically defined chromosomally abnormal single cells and whole-genome amplified products, previously analysed by array-CGH, selected from 68 clinical PGT-A cycles with embryos biopsied at cleavage stage. NGS specificity for aneuploid embryo call was 100% with a sensitivity of 100%. Fiorentino concluded that this product demonstrated a robust methodology ready for clinical applications in reproductive medicine, which potential advantages of reduced costs and enhanced precision.
Improves IVF outcomes
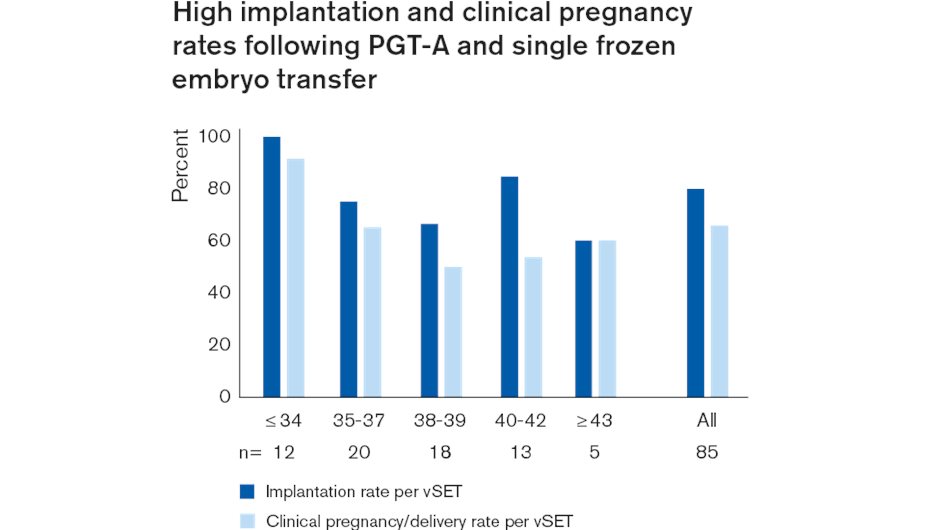
PGT-A, (Preimplantation Genetic Testing for Aneuploidy), has been shown to improve implantation and clinical pregnancy rates and reduce miscarriage rates in several, mainly single centre, randomised control studies (RCTs)11-14. Recently, a large RCT, involving 34 clinics and 9 genetic testing labs, the Single Embryo TrAnsfeR of Euploid Embryo (STAR) Study, using the VeriSeq PGS Solution showed a significant improvement in live birth rates per embryo transfer in women between the ages of 35 and 40 years. This confirms a similar effect in the US SART registry showing a significant benefit in this age range.
Combined with ‘freeze all’ blastocysts by vitrification and replacement of warmed euploid blastocysts in later managed cycles, NGS based PGT-A is a highly effective strategy for embryo selection resulting in high implantation rates at all ages, facilitating elective single embryo transfer.15 See the graph below.
VeriSeq PGS kit
The VeriSeq PGS Solution is a fully-kitted solution designed for multiplexing up to 24 samples per run on the MiSeq System.
- Industry-leading data quality: More than 90% of the world’s sequencing data is generated by Illumina sequencing by synthesis (SBS) chemistry*
- Fast, streamlined workflow: Sample to answer in approximately 12 hours
- Low input: NGS offers a highly sensitive method for screening embryos, requiring as little as 1 ng of DNA from a SurePlex DNA amplification reaction.
- DNA can be obtained from a blastomere biopsy, from a day 3 embryo, or from a trophectodermal (TE) biopsy from a blastocyst
PGT-M
Preimplantation genetic testing for Monogenic Disease (PGT-M) assesses embryos to help prevent the transmission of an inherited genetic disorder to children.
Improve outcomes by better embryo selection
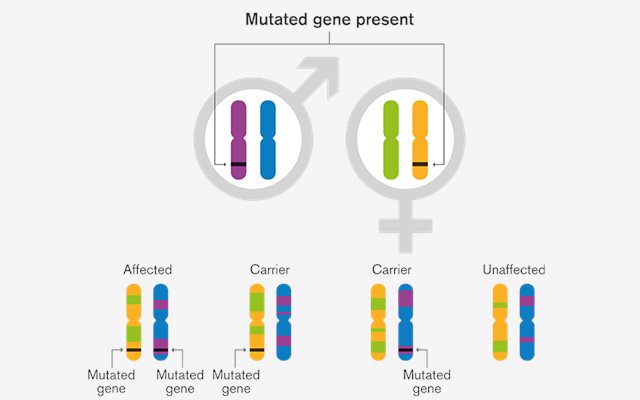
Couples may know that they are a carrier of a genetic disorder because they already have an affected child or they may be aware of a family history of the disorders or may have had their DNA tested. For these couples there is a chance that they could have a child who is affected by this genetic disorder. Using Preimplantation Genetic Testing for Monogenic Disorders (PGT-M) it is possible to avoid passing on that genetic disorders to their offspring. The technique works by screening the embryos for the disorders before implantation in the womb. In order to carry out PGT-M, in vitro fertilisation is essential so that several embryos can be produced and tested to find out which ones are suitable for transfer.
Preimplantation genetic testing for Monogenic Disease (PGT-M)
By identifying IVF embryos that most likely do not carry a specific genetic disorder, PGT-M can enable the transfer of embryos most likely to be unaffected and reduce a genetically at-risk couple’s chances of passing a known genetic disorder to their offspring.
Karyomapping: A comprehensive genome-wide test for PGT-M
A blood samples is taken from the father, the mother and a close relative of known disease status (called reference). The technique of Karyomapping looks at the chromosomes of the mother, father and the reference at approximately 300,000 different points and finds a DNA fingerprint unique to the chromosome that carries the defective gene. Using IVF, embryos are tested to reveal those that have inherited the affected chromosome and those that have inherited normal copies of the gene and likely to be free of the disorder. Embryo that are considered free of the disorder are transfer to the mother’s uterus.
Karyomapping is highly accurate
Natesan et al., (2014) reported the validation of karyomapping in a multi-centre study in which parental, reference and 2018 embryos samples from 44 PGT-M cases were genotyped retrospectively for ~300,000 genome-wide SNP loci. Each sample was karyomapped, the disease status was analysed blind and the results were compared for concordance with the original diagnosis based on targeted haplotyping with direct mutation detection. The result showed that karyomapping is highly accurate and facilitate the inheritance of almost any single-gene defect, greatly expanding the range of conditions that PGT-M can be offered clinical without the need for customised test development.
Karyomapping is a highly efficient, accurate and robust method for PGT-M of single gene disorders
Konstantinidis et al., (2015) reported their own validation results and the first prospective clinical application of karyomapping, ongoing pregnancies and live births in a series of single-gene defect cases. The results presented in the study indicated that karyomapping is a highly efficient, accurate and robust method for PGT-M of single gene disorders. In addition they confirm that the principle advantage of karyomapping is a significant reduction in the time and effort required to prepare for a PGT-M cycle, which for karyomapping only involves genotyping the parents and child or relative to confirm that is sufficient informative SNP coverage in a particular family and single-gene defect locus.
HumanKaryomap-12 DNA Analysis Kit
The HumanKaryomap-12 DNA Analysis Kit is a comprehensive genome-wide test available at the single-cell level. It provides insight into the inheritance of single-gene defects. This BeadChip array targets ~300,000 of the most informative markers in the genome for efficient genome-wide coverage. Karyomapping uses biomarkers within the genome to assess the likelihood of an embryo carrying a gene variant involved in a single-gene disorder.
For Research Use Only. Not for use in diagnostic procedures.
References
- 1. Liu J, Wang W, Sun X, et al. DNA microarray reveals that high proportions of human blastocysts from women of advanced maternal age are aneuploid and mosaic. Biol Reprod. 2012;87(6):148.elihood of an embryo carrying a gene variant involved in a single-gene disorder.
- 2. Ata B, Kaplan B, Danzer H, et al. Array CGH analysis shows that aneuploidy is not related to the number of embryos generated. Reprod Biomed Online. 2012;24(6):614-620.
- 3. Harton GL, Munné S, Surrey M, et al; for the PGD Practitioners Group. Diminished effect of maternal age on implantation after preimplantation genetic diagnosis with array comparative genetic hybridization. Fertil Steril. 2013;100(6):1695-1703.
- 4. Simpson JL. Causes of fetal wastage. Clin Obstet Gynecol. 2007;50(1):10-30.
- 5. Scott RT Jr, Ferry K, Su J, Tao X, Scott K, Treff NR. Comprehensive chromosome screening is highly predictive of the reproductive potential of human embryos: a prospective, blinded, nonselection study. Fertil Steril. 2012;97(4):870-875.
- 5. Scott RT Jr, Ferry K, Su J, Tao X, Scott K, Treff NR. Comprehensive chromosome screening is highly predictive of the reproductive potential of human embryos: a prospective, blinded, nonselection study. Fertil Steril. 2012;97(4):870-875.
- 6. Liu J, Wang W, Sun X, et al. DNA microarray reveals that high proportions of human blastocysts from women of advanced maternal age are aneuploid and mosaic. Biol Reprod. 2012;87(6):148.
- 7. Forman EJ, Hong KH, Ferry KM, et al. In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertil Steril. 2013;100(1):100-107.
- 8. Yang Z, Liu J, Collins GS, et al. Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Mol Cytogenet. 2012;5(1):24.
- 9. Grifo JA, Hodes-Wertz B, Lee HL, Amperloquio E, Clarke-Williams M, Adler A. Single thawed euploid embryo transfer improves IVF pregnancy, miscarriage, and multiple gestation outcomes and has similar implantation rates as egg donation. J Assist Reprod Genet. 2013;30(2):259–26
- 10. Fiorentino F, Biricik A, Bono S, Spizzichino L, Cotroneo E, Cottone G, Kokocinkski F and Michel CM (2014) Development and validation of a next-generation sequencing-based protocol for 24-chromosome aneuploidy screening of embryos. Fertility and Sterility Vol 101, Issue 5, Pages 1375-1382 e2
- 11. Forman EJ, Hong KH, Ferry KM, Tao X, Taylor D, Levy B, Treff NR and Scott RT (2013) In vitro fertilization with single euploid blastocyst transfer: a randomized controlled trial. Fertility and Sterility 100 100–7.e1.
- 12. Rubio C, Bellver J, Rodrigo L, Castillón G, Guillén A, Vidal C, Giles J, Ferrando M, Cabanillas S, Remohí J et al. (2017) In vitro fertilization with preimplantation genetic diagnosis for aneuploidies in advanced maternal age: a randomized, controlled study. Fertility and Sterility 107 1122–1129.
- 13. Scott RT, Upham KM, Forman EJ, Hong KH, Scott KL, Taylor D, Tao X and Treff NR (2013) Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertility and Sterility 100 697–703.
- 14. Yang Z, Liu J, Collins GS, Salem SA, Liu X, Lyle SS, Peck AC, Sills ES and Salem RD (2012) Selection of single blastocysts for fresh transfer via standard morphology assessment alone and with array CGH for good prognosis IVF patients: results from a randomized pilot study. Molecular Cytogenetics 5 24.
- 15. Gorodeckaja J, Neumann S, McCollin A, Ottolini CS, Wang J, Ahuja K, Handyside A and Summers M (2019) High implantation and clinical pregnancy rates with single vitrified-warmed blastocyst transfer and optional aneuploidy testing for all patients. Human Fertility (Cambridge, England) 1–12.
- 16. Handyside AH, Harton GL, Mariani B, Thornhill AR, Affara N, et al. (2010) Karyomapping: A universal method for genome wide analysis of genetic disease based on mapping crossovers between parental haplotypes. J Med Genet. 47: 651–658